C9orf72 Gene Hexanucleotide Repeat Expansion
Summary and Pricing 
Test Method
Combination Of Repeat-Primed PCR and Fluorescent Fragment-Length Assay| Test Code | Test Copy Genes | Test CPT Code | Gene CPT Codes Copy CPT Code | Base Price | |
|---|---|---|---|---|---|
| 151 | C9orf72 | 81479 | 81479 | $350 | Order Options and Pricing |
An additional 25% charge will be applied to STAT orders. STAT orders are prioritized throughout the testing process.
Turnaround Time
3 weeks on average for standard orders or 2 weeks on average for STAT orders.
Please note: Once the testing process begins, an Estimated Report Date (ERD) range will be displayed in the portal. This is the most accurate prediction of when your report will be complete and may differ from the average TAT published on our website. About 85% of our tests will be reported within or before the ERD range. We will notify you of significant delays or holds which will impact the ERD. Learn more about turnaround times here.
Targeted Testing
For ordering sequencing of targeted known variants, go to our Targeted Variants page.
Clinical Features and Genetics 
Clinical Features
Amyotrophic lateral sclerosis (ALS) is a neurodegenerative disease characterized by a selective loss of motor neurons in the motor cortex, brain stem, and spinal cord (Tandan and Bradley. 1985. PubMed ID: 4051456). The dysfunction and loss of these neurons results in rapid progressive muscle weakness, atrophy and ultimately paralysis of limb, bulbar and respiratory muscles. The mean age of onset of symptoms is about 55 years of age; most cases begin between 40 and 70 years of age. The annual incidence of ALS is 1-2 per 100,000 (Cleveland and Rothstein. 2001. PubMed ID: 11715057).
The most common initial symptoms include twitching and cramping of muscles of the hands and feet, loss of motor control in the hands and arms, general muscle weakness, rapid fatigue, and tripping and falling. The disease usually begins with asymmetric involvement of the muscles. As the disease progresses, symptoms may include difficulty in talking, breathing and swallowing, and paralysis. Cognitive impairment has not been initially associated with ALS. However, frontotemporal dementia (FTD) has been reported in several cases. Dementia has been documented in patients with ALS from different ethnic groups and affects both males and females (Wikström et al. 1982. PubMed ID: 7125994; Lipton et al. 2004. PubMed ID: 15351890; Mitsuyama and Inoue. 2009. PubMed ID: 19780984).
Genetics
About 10% of ALS cases are familial (Emery and Holloway. 1982. PubMed ID: 7180680). In most of these families, ALS is inherited in an autosomal dominant manner (AD-ALS) with age-dependent penetrance. In rare families, the disease is transmitted in an autosomal recessive or dominant X-linked pattern. About 90% of patients with ALS are simplex cases (SALS) with no known affected relatives. It is unclear how many of the apparently simplex cases are inherited with low penetrance.
The clinical presentations of familial ALS (FALS) and sporadic ALS (SALS) are similar. However, the onset of symptoms in FALS is usually earlier compared to that of SALS (Kinsley and Siddique. 2015. PubMed ID: 20301623).
Autosomal Dominant ALS (AD-ALS) is a clinically and genetically heterogeneous disorder that affects all ethnic groups. Several genes are involved including C9orf72, SOD1, FUS, TARDBP, ANG, OPTN and VCP.
An expansion of a GGGGCC hexanucleotide repeat in a non-coding region of the C9orf72 gene was reported in patients with ALS with or without FTD (Renton et al. 2011. PubMed ID: 21944779; DeJesus-Hernandez et al. 2011. PubMed ID: 21944778). Various size ranges of both normal and pathogenic alleles have been reported (Human Gene Mutation Database). Alleles with less than 25 repeats are usually considered normal (Majounie et al. 2012. PubMed ID: 22406228; van der Zee et al. 2013. PubMed ID: 23111906). Alleles with more than 30 repeats are considered pathogenic (Renton et al. 2011. PubMed ID: 21944779).
This expanded repeat appears to be a major genetic cause of ALS accounting for up to 40% of FALS cases and 5% of SALS cases (Byrne et al. 2012. PubMed ID: 22305801). The GGGGCC hexanucleotide repeat expansion was reported in patients with ALS, ALS-FTD or FTD (Kinsley and Siddique. 2015. PubMed ID: 20301623).
The function of C9orf72 is unknown at this time. However, nuclear RNA foci were detected in affected tissues of patients with expanded GGGGCC repeats, suggesting defective RNA processing (Rademakers et al. 2012. PubMed ID: 22732773).
Clinical Sensitivity - Repeat-Primed PCR & Fragment Length
This test may detect a pathogenic hexanucleotide repeat expansion in up to 40% of FALS cases and 5% of SALS cases (Byrne et al. 2012. PubMed ID: 22305801).
Testing Strategy
A combination of amplicon-length analysis and repeat-primed PCR is used as a screening method for the presence or absence of a pathogenic GGGGCC hexanucleotide repeat expansion located in the first intron of C9orf72 (Akimoto et al. 2014. PubMed ID: 24706941). Two repeat-primed PCR assays, for the 3’ and 5’ ends of the repeat region, are used as described by Cleary et al. (Cleary et al. 2016. PubMed ID: 27288208). The amplicon-length analysis is used as described by DeJesus-Hernandez et al (DeJesus-Hernandez et al. 2011. PubMed ID: 21944778). Test controls included DNA samples from individuals known to have the repeat expansion, individuals with intermediate alleles, and healthy individuals.
This test is designed to only detect pathogenic expansions of a GGGGCC hexanucleotide repeat in a non-coding region of the C9orf72 gene.
Indications for Test
All patients with symptoms suggestive of ALS, including AD-ALS and SALS, with or without FTD are candidates for this test.
All patients with symptoms suggestive of ALS, including AD-ALS and SALS, with or without FTD are candidates for this test.
Gene
| Official Gene Symbol | OMIM ID |
|---|---|
| C9orf72 | 614260 |
| Inheritance | Abbreviation |
|---|---|
| Autosomal Dominant | AD |
| Autosomal Recessive | AR |
| X-Linked | XL |
| Mitochondrial | MT |
Disease
| Name | Inheritance | OMIM ID |
|---|---|---|
| Frontotemporal Dementia And/Or Amyotrophic Lateral Sclerosis | AD | 105550 |
Citations 
- Akimoto et al. 2014. PubMed ID: 24706941
- Byrne et al. 2012. PubMed ID: 22305801
- Cleary et al. 2016. PubMed ID: 27288208
- Cleveland and Rothstein. 2001. PubMed ID: 11715057
- DeJesus-Hernandez et al. 2011. PubMed ID: 21944778
- Emery and Holloway. 1982. PubMed ID: 7180680
- Human Gene Mutation Database (Bio-base).
- Kinsley and Siddique. 2015. PubMed ID: 20301623
- Lipton et al. 2004. PubMed ID: 15351890
- Majounie et al. 2012. PubMed ID: 22406228
- Mitsuyama and Inoue. 2009. PubMed ID: 19780984
- Rademakers et al. 2012. PubMed ID: 22732773
- Renton et al. 2011. PubMed ID: 21944779
- Tandan and Bradley. 1985. PubMed ID: 4051456
- van der Zee et al. 2013. PubMed ID: 23111906
- Wikström et al. 1982. PubMed ID: 7125994
Ordering/Specimens 
Ordering Options
We offer several options when ordering sequencing tests. For more information on these options, see our Ordering Instructions page. To view available options, click on the Order Options button within the test description.
myPrevent - Online Ordering
- The test can be added to your online orders in the Summary and Pricing section.
- Once the test has been added log in to myPrevent to fill out an online requisition form.
- PGnome sequencing panels can be ordered via the myPrevent portal only at this time.
Requisition Form
- A completed requisition form must accompany all specimens.
- Billing information along with specimen and shipping instructions are within the requisition form.
- All testing must be ordered by a qualified healthcare provider.
For Requisition Forms, visit our Forms page
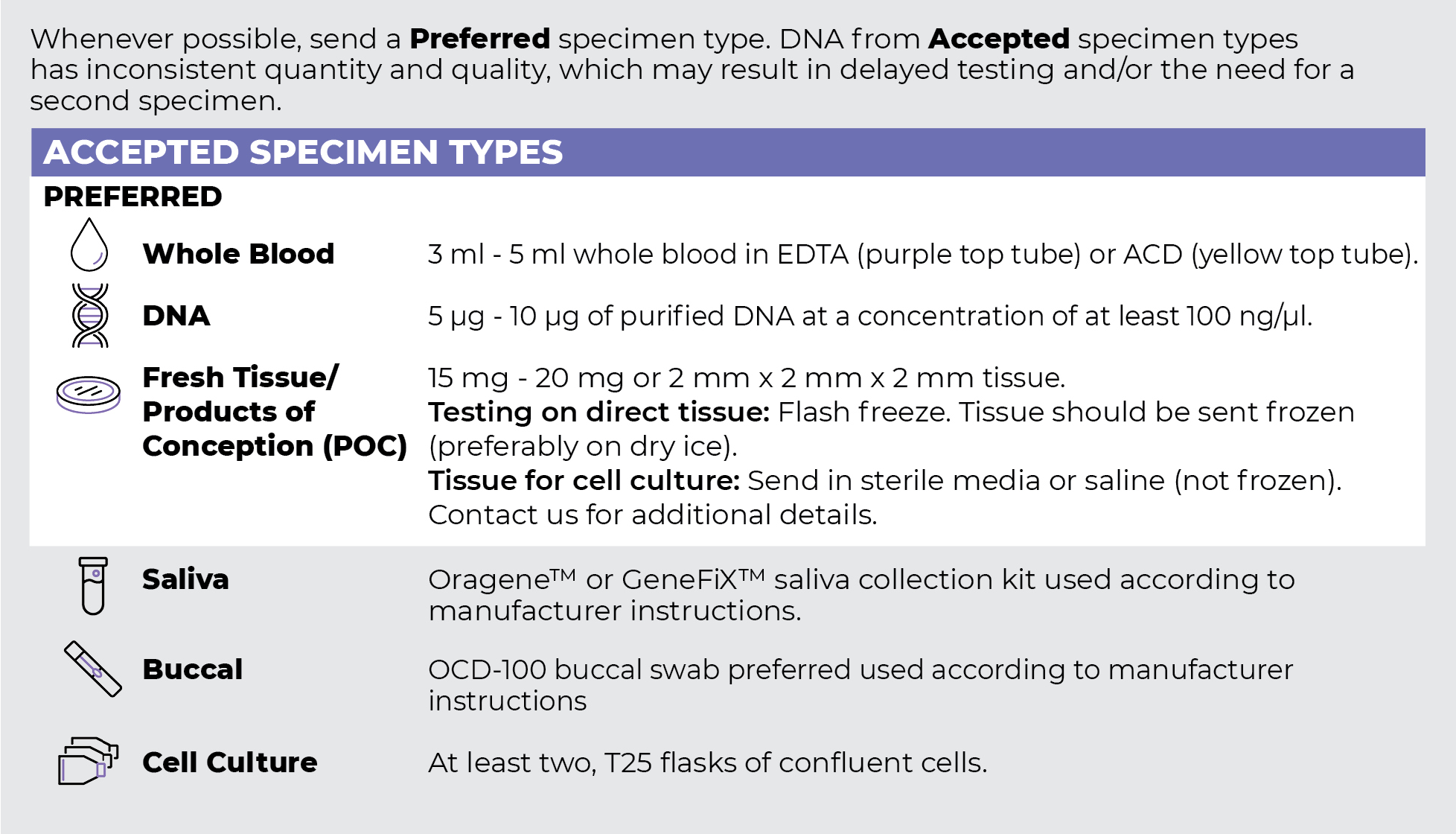
Specimen Types
Specimen Requirements and Shipping Details
ORDER OPTIONS
View Ordering Instructions1) Select Test Type
2) Select Additional Test Options
No Additional Test Options are available for this test.

