Updated Intellectual Disability, Epilepsy, and Autism (IDEA) Genetic Testing Panel
Published on
Intellectual disability (ID), epilepsy, and autism spectrum disorders (ASDs) are a heterogeneous group of neurodevelopmental disorders that are inherited in a multifactorial fashion. With an extensive list of genes that are associated with ID, epilepsy, and ASD phenotypes, PreventionGenetics' Intellectual Disability, Epilepsy, and Autism (IDEA) Panel is the best alternative to whole exome sequencing. Through work with collaborators and frequent panel updates, the IDEA comprehensive panel includes genes at the forefront of autism, intellectual disability, and epilepsy research.
Why Genetic Testing?
ASD and related conditions are often part of a broader medical condition, with clinical consequences beyond the defined phenotype. Genetic testing can potentially inform treatment or access to necessary support services. By performing genetic testing in the context of ID, epilepsy, and ASD, enhancement of patient care can be achieved through the identification of previously unrecognized comorbidities and the active surveillance and early intervention before symptoms develop.
- 70% of individuals with an ASD diagnosis have a comorbidity (Sztainberg & Zoghbi 2016. PubMed ID: 27786181).
- Distinct neurodevelopmental phenotypes are frequently associated with epilepsy, cardiac defects, and renal anomalies
- The genetic cause may implicate a specific biological mechanism or cognitive/behavioral profile.
- Knowledge of genetic etiology can inform pharmacotherapy and direct access to support services.
Test Details
PreventionGenetics’ Intellectual Disability, Epilepsy, and Autism (IDEA) Panel is exome-based and allows for reflex to PGxome whole exome sequencing. The IDEA panel also includes exome-wide copy number variant (CNV) detection. This method allows for cost-effective identification and reporting of potentially important, large CNVs across the full exome in conjunction with any sequencing variants and/or smaller CNVs identified within the panel. CNV detection via exome sequencing is equally or more sensitive than chromosomal microarray (CMA). This newer option provides the opportunity to bypass standard CMA testing (Reddy et al. 2012; Sahoo et al. 2016).
“I’m glad we didn’t start with CMA (for my patient). We probably would have stopped after finding the deletion and missed the other two diagnoses.” –Healthcare Provider in Colorado
The Intellctual Disability, Epilepsy, and Autism (IDEA) Panel is primarily indicated for patients with ID, epilepsy, and/or ASDs who are negative for Fragile-X syndrome. The test is billed as a panel and offers an average turnaround time of 28 days.
| Pricing | |
| Family Trio | $2,290 |
| Family - Duo | $1,790 |
| Patient Plus | $1,790 |
| Patient Only | $1,690 |
Reporting
Clinical features are used to identify relevant sequence variants:
1. In genes known to be associated with the patient’s phenotype.
2. In genes possibly associated with the patient’s phenotype.
Secondary findings or carrier status variants are not reported as part of the ASD-ID & epilepsy analysis.
Diagnostic Yields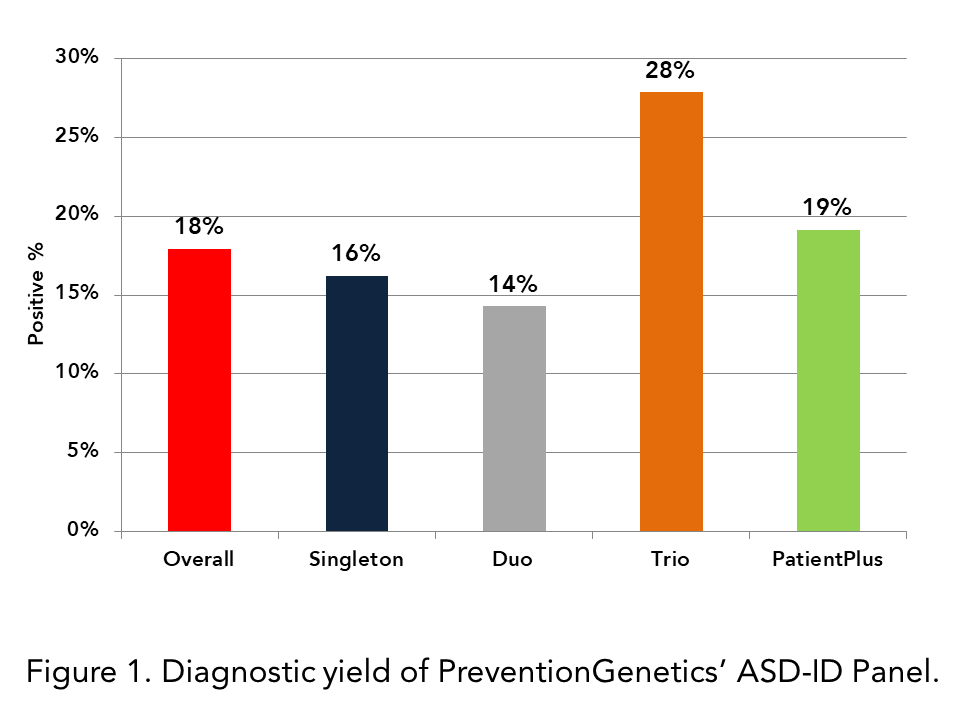
- The percentage of all IDEA panel tests with a positive finding, including CNVs, is approximately 18%.
- The diagnostic yields of Duo and PatientPlus analyses are 14% and 19%, respectively. The diagnostic yield of Trio testing is 28%, considerably higher.
- Since 2018, 15% of ASD-ID cases have had a confirmed deletion or duplication event identified via NGS that was relevant to the patient’s phenotype. In many cases, this event was not identified by initial CMA analyses.
- Trio diagnostic rates at PreventionGenetics correspond closely to the diagnostic rate of ASD-ID exome testing reported in the literature (Bourgeron. 2015. PubMed ID: 26289574; Bowling et al. 2017. PubMed ID: 28554332; Snoeijen-Schouwenaars et al. 2019. PubMed ID: 30525188; Woodbury-Smith and Scherer. 2018. PubMed ID: 29574884).
Finding Answers for Patients: Two Cases
| Case 1 | Case 2 | |
|---|---|---|
| Demographics | 9 year-old female | 21 year-old male |
| Clinical Features |
delayed milestones in childhood autistic features hypertelorism retrognathia micrognathia myopia intellectual disability telecanthus bilateral ptosis recurrent otitis media eczema |
global developmental delay autism spectrum disorder intellectual disability joint hypermobility delayed speech and language development receptive language difficulty tantrums anxiety deeply-set eyes long face myopathic facies abnormal muscle tone |
| Previous Testing | Negative CMA | Negative CMA in 2001 at age five |
| Results and Diagnosis | de novo missense variant in ACTB Diagnosis of (AD) Baraitser-Winter Syndrome |
15q11q13 duplication via NGS (confirmed by aCGH) Diagnosis of 15q Duplication Syndrome |
| Actionable Clinical Intervention | Screening for congenital cardiac disease, bilateral hydronephrosis, B-cell lymphoma speech therapy, physical therapy | Seizure management, treatment of behavioral manifestations, heart defects (>25% of cases), genitourinary defects (25-75% of cases), muscular hypotonia |
| Related Tests | |
| Autism Spectrum Disorders Panel | |
| X-Linked Intellectual Disability Panel | |
| Top 99 Genetic Causes of Developmental Delay Panel | |
| Comprehensive Epilepsy and Seizure Panel | |
| Chromosomal Microarray | |
References:
Bourgeron. 2015. Nature Reviews Neuroscience. 16: 9. PubMed ID: 26289574
Bowling et al. 2017. Genome Medicine. 9: 43. PubMed ID: 28554332
Karam S.M. et al. 2015. American Journal of Medical Genetics. Part A. 167: 1204-14. PubMed ID: 25728503
Larsen E. et al. 2016. Molecular Autism. 7: 44. PubMed ID: 27790361
Reddy et al. 2012. PubMed ID: 22467168
Sahoo et al. 2016. PubMed ID: 27337029
Snoeijen-Schouwenaars et al. 2019. Epilepsia. 60: 1. PubMed ID: 30525188
Sztainberg & Zoghbi. 2016. Nature Neuroscience. 19: 11. PubMed ID: 27786181
Vorstman et al. 2017. Nature Reviews Genetics. 18: 6. PubMed ID: 28260791
Woodbury-Smith and Scherer. 2018. Developmental Medicine & Child Neurology. 60: 5. PubMed ID: 29574884



